WATER SOFTENERS - WHAT IT IS AND HOW THEY WORK
Posted on: 05/13/21Softeners significantly reduce water hardness and are ideal for areas with a lot of salts such as islands.
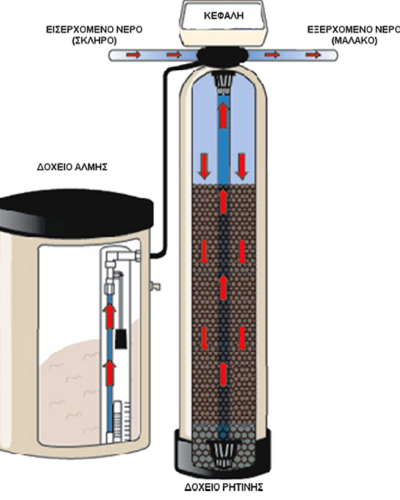
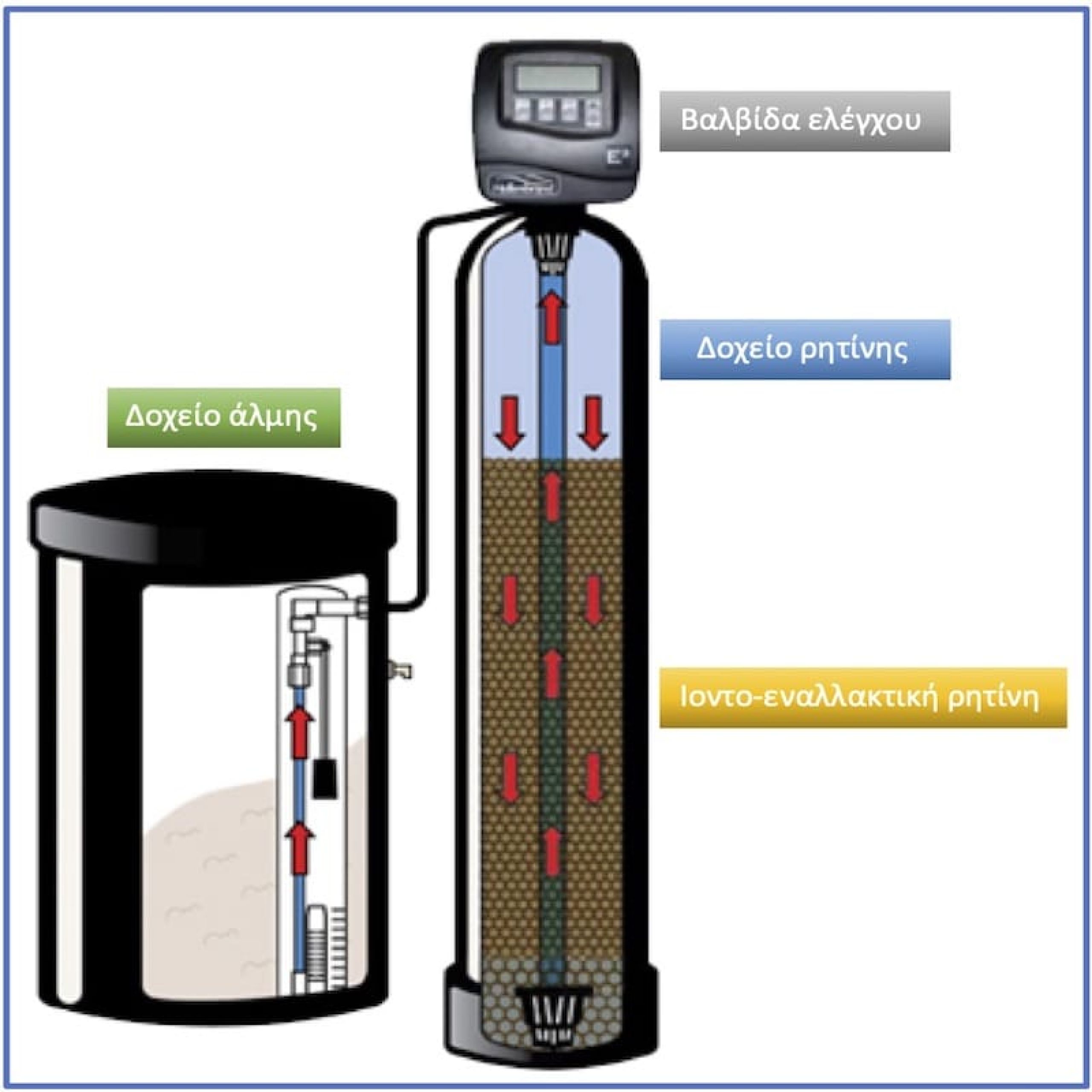
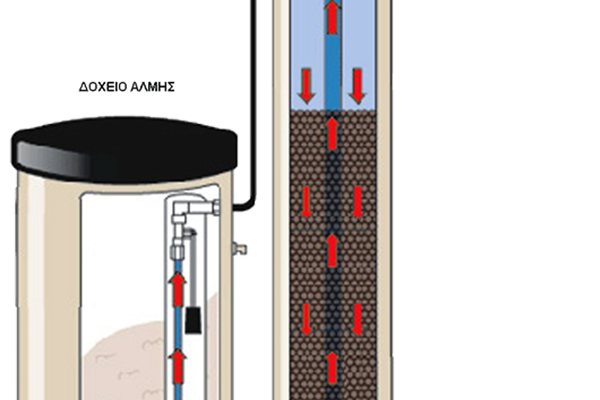
They use a material called resin, which exchanges magnesium and calcium ions for sodium and potassium ions.
When at some point its action is exhausted then it must make a "rebirth". To do this it needs to be washed with sodium chloride, ie coarse salt.
They have an electronic head with which the system can be adjusted to regenerate at a time or when certain liters of water have passed.
Softening is necessary because:
1. Hard water contains calcium and magnesium, as well as other metals and salts that form deposits, thereby reducing the efficiency of appliances that come in contact with hard water.
2. Use less soap and soft water cleaning products.
3. The accumulation of salts from hard water can clog pipes, reduce water flow and reduce the life of electrical appliances, such as water heaters, dishwashers and coffee makers, taps, etc.
4. Hardness can also create an unwanted taste in drinking water, in cooked foods with vegetables.